A longstanding unmet medical need...
Pain and inflammation are a cause, consequence and contributor to a wide range of health disorders. Current solutions are beset by complications and limitations. Aside from NSAIDs, there are two main classes of drugs used to treat pain and inflammation: Opioids and corticosteroids (the latter often referred to as “steroids”). Both carry serious risks and therapeutic constraints.
As is well-known, opioids are at the center of a public health crisis, with physicians, pharmacists and policymakers attempting to steer patients away from these abuse-prone, dangerous substances. No more effective than NSAIDs for most patients, opioids are only suitable for acute pain and do not address inflammation. Corticosteroids also pose a range of risks, including bone loss, vision impairment, infection, and blood sugar disruption. In addition, they do not address pain unless it is caused by inflammation itself.
The world requires safer, non-addictive medicines for pain and inflammation.
Pain and Inflammation: The Therapeutic Challenge
As a key feature of human physiology, pain typically serves as a signal that a body part has sustained damage. Often termed “nociceptive pain”, it is triggered at the site of injury primarily by prostaglandin production, and is regulated by cyclooxygenase (COX) enzymes, comprising two subtypes: COX-1 and COX-2 (NSAIDs function by inhibiting COX enzymes). The biological purpose of inflammation is to enable efficient and rapid cellular repair. However, many types of inflammation—especially when arising from long-term conditions like osteoarthritis—can cascade into a medical problem in themselves, leading to further tissue damage, fever and chronic pain.
NSAIDs: Effective … but with an enduring safety problem
The NSAID category includes aspirin and well-known consumer brands like Advil (ibuprofen) and Aleve (naproxen), as well as prescription-only medicines like celecoxib (Celebrex), ketoprofen, ketorolac and meloxicam. Given their ability to reduce both pain and inflammation, NSAIDs’ non-addictive nature and well-established efficacy have made them drugs of choice for physicians and consumers, with nearly a billion tablets taken worldwide each day.
However, NSAIDs also cause clinically significant gastrointestinal ulcers and bleeding in approximately 25% of users, a problem that has resisted solution since aspirin was introduced more than 100 years ago. As first documented by Dr John L. Wallace and his colleagues in 1990, the root of this problem is that NSAIDs impair the body’s ability to maintain the mucosal layer that ordinarily protects the lining of the digestive organs. This issue is highly consequential—adverse reactions to NSAIDs are a leading cause of hospital deaths. Several approaches have been attempted to address this widespread problem, but all have proved unsatisfactory.
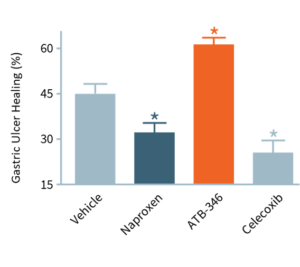
The accompanying bar chart illustrates that gastric ulcers that are left alone will begin to heal. NSAIDs, however, in addition to their tendency to cause ulcers throughout the gastrointestinal tract, also can retard their healing.
In this experiment, ulcers were induced in animals that were then treated for four days. With no drug treatment (“Vehicle”), ulcers decreased in size by about 45%. Typically, NSAIDs (naproxen, celecoxib) significantly impair ulcer healing, whereas ATB-346 significantly accelerated ulcer healing. We believe that this experiment is the first instance of an NSAID demonstrating promise as an ulcer healing agent. ATB-346 has also shown the potential to improve healing in body systems, including bone healing.
COX-2 selective NSAIDs
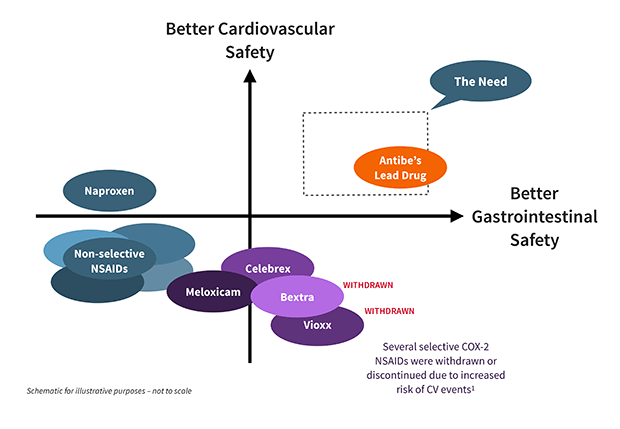
While traditional NSAIDs block both COX-1 and COX-2 enzymes, the late 1990s saw the introduction of NSAIDs that specifically inhibit COX-2, on the premise that stomach damage was primarily due to the effects of suppressed COX-1. Although COX-2 selective NSAIDs were outstandingly successful commercially, the FDA eventually removed the majority from the market (e.g., Vioxx, Bextra), due to increased risk of cardiovascular events. A few COX-2 selective NSAIDs, principally celecoxib, continue to be available. To counteract cardiovascular concerns, they are often administered with low-dose aspirin, nullifying their gastroprotective advantage—while intensifying damage to the small intestine.*
……………………….
* Damage to most of the small intestine was hidden from researchers until the recent availability of capsule endoscopy, a method of viewing the digestive tract via a swallowed capsule that contains a miniaturized camera. Traditional endoscopes are only able to inspect the stomach/duodenum or the large intestine.
……………………….
Enteric coatings
Enterically-coated NSAIDs were introduced to prevent the digestion of the NSAID tablet by the stomach, thereby protecting that organ from damage. However, these coatings necessarily deliver intact pills (now absent their protective coating) into the more easily-damaged small intestine. As such, they can cause more serious injury than they prevent. Moreover, enteric coatings reduce the efficacy of NSAIDs, necessitating increased dosages to achieve intended therapeutic effects.
Proton-pump inhibitors (PPIs) and H2 antagonists
PPIs and H2 antagonists (e.g., esomeprazole and ranitidine; branded as Nexium and Zantac) are often co-prescribed with NSAIDs to protect the stomach. However, this treatment strategy dramatically increases intestinal bleeding, ulcers, and risk of perforation, as observed in rising rates of hospitalization and mortality. Notably, reduced stomach acidity can enable the survival of dangerous microbes, including C. difficile, with resulting opportunistic infections compounding the damage caused by the NSAIDs themselves.
Polypharmacy complications
The under-appreciated complications arising from using several drugs concurrently (known as “polypharmacy”) are relevant to many NSAID users and their physicians. As noted earlier, the co-administration of low-dose aspirin with COX-2 selective NSAIDs obviates their stomach-protective function, while compounding risks to the small intestine. Many patients suffering from pain and unwanted inflammation are also in treatment for conditions like cardiovascular disease and gastrointestinal ulcers, creating a situation where today’s NSAIDs would be useful but are largely contraindicated. (As noted above, Antibe’s drugs both prevent ulcers and also improve healing of existing ulcers.)
NSAIDs: Effective … but with an enduring safety problem
The NSAID category includes aspirin and well-known consumer brands like Advil (ibuprofen) and Aleve (naproxen), as well as prescription-only medicines like celecoxib (Celebrex), ketoprofen, ketorolac and meloxicam. Given their ability to reduce both pain and inflammation, NSAIDs’ non-addictive nature and well-established efficacy have made them drugs of choice for physicians and consumers, with nearly a billion tablets taken worldwide each day.
However, NSAIDs also cause clinically significant gastrointestinal ulcers and bleeding in approximately 25% of users, a problem that has resisted solution since aspirin was introduced more than 100 years ago. As first documented by Dr John L. Wallace and his colleagues in 1990, the root of this problem is that NSAIDs impair the body’s ability to maintain the mucosal layer that ordinarily protects the lining of the digestive organs. This issue is highly consequential—adverse reactions to NSAIDs are a leading cause of hospital deaths. Several approaches have been attempted to address this widespread problem, but all have proved unsatisfactory.
As depicted in the accompanying chart, intestinal damage in rats caused by naproxen (dark blue bars) and celecoxib (light blue bars) is worsened significantly when omeprazole (“Omep”), a common PPI, or low-dose aspirin (for cardiovascular protection), is given at the same time. These results have been replicated in human studies. When co-administered with PPIs, ATB-346 remained gastrointestinal-safe (although there is no need for PPIs or other acid-reduction medication to be co-administered with ATB-346) .
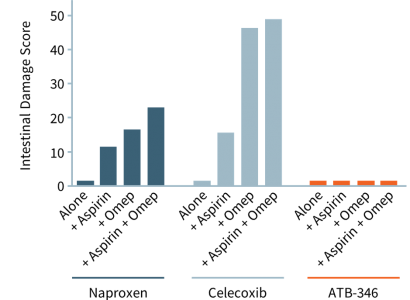
Unlike Other NSAIDs, ATB-346 Is Gastrointestinal-safe, Even When Administered With Aspirin
Current Solutions Are Unsatisfactory...
Overall, intestinal injury is more difficult to diagnose and treat than stomach damage, and symptoms correlate poorly with the severity of tissue injury, complicating medical assessment. By focusing on the stomach, physicians may be inadvertently placing their patients at risk of serious, difficult-to-diagnose injury—with no proven-effective therapies and significantly higher rates of morbidity and mortality. Moreover, many current solutions do not even adequately protect the stomach.
