ATB-340 is a gastrointestinal-safe version of low-dose aspirin, a drug taken daily by millions of people for cardiovascular protection.
For decades, low-dose aspirin has been known to provide a significant reduction in the risk of stroke and, more recently, to provide a dramatic reduction in the risk of digestive system cancers. However, aspirin, like other NSAIDs, causes stomach ulcers and intestinal bleeding in a significant portion of the population, precluding its broad prescription by physicians. ATB-340 is a hydrogen sulfide-releasing derivative of aspirin that has been shown to be gastrointestinal-safe. Academic proof-of-concept studies for ATB-340 are underway.
ATB-340: A Gastrointestinal-safe Anti-thrombotic
Aspirin produces its anti-thrombotic actions by inhibiting the COX-1 enzyme in platelets. However, aspirin causes significant bleeding and ulcers in the rat stomach.1 In preclinical studies, ATB-340 exhibits equivalent inhibition of COX-1 but without gastrointestinal damage.
As with other NSAIDs, aspirin triggers gastric damage by causing white blood cells (leukocytes) to stick to the inner surface of blood vessels in the stomach, contributing significantly to ulcer formation. Current medical practice is to combine aspirin with an acid reduction drug (e.g., a proton pump inhibitor such as esomeprazole), protecting the stomach but increasing damage to the small intestine. ATB-340 demonstrates markedly reduced leukocyte adherence in rats compared to aspirin.
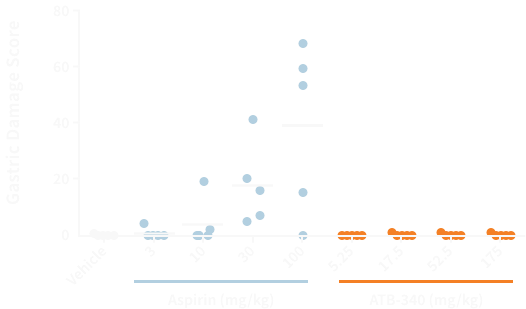
Aspirin, but not ATB-340, causes significant gastric damage in rats.
Nitric Oxide. 2015 Apr 30;46:25-31.
